Hippocampal ZnT3 (SLC30A3) Levels Reflect Hippocampal Tissue Damage in Chronic Exercising Diabetic Rats
DOI:
https://doi.org/10.58600/eurjther1874Keywords:
Chronic exercise, diabetes, MDA, hippocampus, ZnT3Abstract
Objective: In this study, it was investigated how chronic exercise affects hippocampus tissue damage and ZnT3 levels in diabetic rats.
Methods: The 40 adult rats wereused in the study were divided into 4 equal groups: Control (G1), Exercise Control (G2), Diabetes (G3), Diabetes+Exercise (G4). Diabetes was induced in animals in G3 and G4 by injecting intraperitoneal streptozotocin (STZ) twice, 24 hours apart. The animals in G2 and G4 were runedon the rat treadmill for 45 minutes daily for 4 weeks. MDA (spectrophotometric method) and ZnT3 (ELISA method) levels were determined in hippocampus tissue samples obtained from animals sacrificed at the end of the experimental procedures.
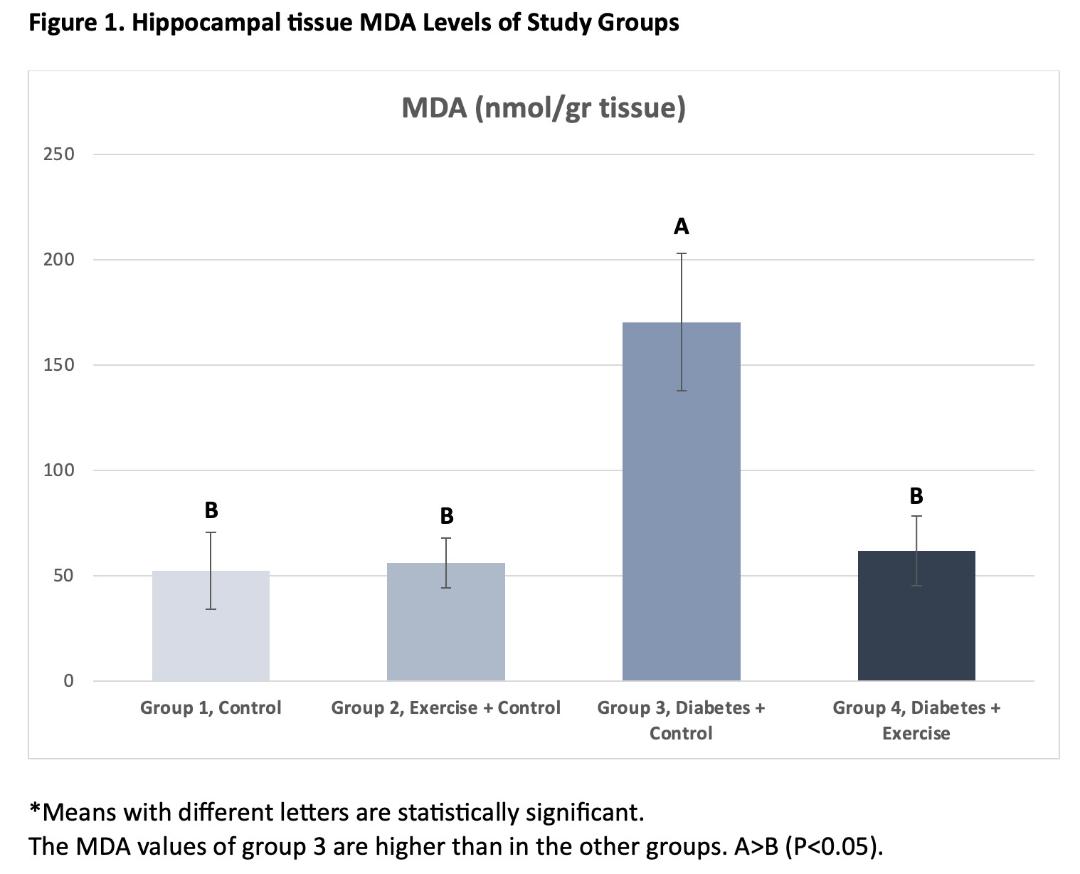
Results: In the current study, the highest MDA and lowest ZnT3 levels in the hippocampus tissue were obtained in the diabetes group (G3) (P<0.05). Chronic exercise prevented increased hippocampal tissue damage in diabetic rats and reversed decreased ZnT3 levels (P<0.05).
Conclusion: The results of our study showed that 4 weeks of chronic exercise could be prevent increased tissue damage in the hippocampus tissue of diabetic rats and ameliorate the decreased ZnT3 levels. The data obtained in this study indicate that ZnT3 levels in diabetic rats may be an indicator of hippocampal tissue damage.
Metrics
References
Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M (2006) Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443(7112):709–712. https://doi:10.1038/nature05162
Kaveh MH, Noori K, Nazari M, Khademi K (2022) Quality of life and metabolic indicators of patients with type 2 diabetes: A cross-sectional study in Iran. Int J Endocrinol 2022:4046012. https://doi:10.1155/2022/4046012
Kowluru RA, Mohammad G (2022) Epigenetic modifications in diabetes. Metabolism 126:154920. https://doi:10.1016/j.metabol.2021.154920
Darenskaya MA, Kolesnikova LI, Kolesnikov SI (2021) Oxidative stress: Pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med 171(2):179-189. https://doi:10.1007/s10517-021-05191-7
Nisar O, Pervez H, Mandalia B, Waqas M, Sra HK (2020) Type 3 Diabetes Mellitus: A link between Alzheimer's disease and Type 2 Diabetes Mellitus. Cureus 12(11):e11703. https://doi:10.7759/cureus.11703
Chornenkyy Y, Wang WX, Wei A, Nelson PT (2019)Alzheimer's disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline.Brain Pathol 29(1):3-17. https://doi:10.1111/bpa.12655
Hamzé R, Delangre E, Tolu S, Moreau M, Janel N, Bailbé D, Movassat J (2022) Type 2 Diabetes Mellitus and Alzheimer's Disease: Shared molecular mechanisms and potential common therapeutic Targets. Int J Mol Sci 23(23):15287. https://doi:10.3390/ijms232315287
Mittal K, Mani RJ, Katare DP (2016) Type 3 Diabetes: Cross talk between differentially regulated proteins of type 2 diabetes mellitus and Alzheimer's disease. Sci Rep 6:25589. https://doi:10.1038/srep25589
Leszek J, Trypka E, Tarasov VV, Ashraf GM, Aliev G (2017) Type 3 Diabetes Mellitus: A novel implication of Alzheimers Disease. Curr Top Med Chem 17(12):1331-1335. https://doi:10.2174/1568026617666170103163403
Sędzikowska A, Szablewski L (2021) Insulin and insulin resistance in Alzheimer's disease. Int J Mol Sci 22(18):9987. https://doi:10.3390/ijms22189987
de la Monte SM, Neusner A, Chu J, Lawton M (2009) Epidemilogical trends strongly suggest exposures as etiologic agents in the pathogenesis of sporadic Alzheimer's disease, diabetes mellitus, and non-alcoholic steatohepatitis. J Alzheimers Dis 17(3):519-529. https://doi:10.3233/JAD-2009-1070
Pasquier F, Boulogne A, Leys D, Fontaine P (2006) Diabetes mellitus and dementia. Diabetes Metab 32(5 Pt 1):403-414. https://doi:10.1016/s1262-3636(07)70298-7
Brown BM, Sohrabi HR, Taddei K, Gardener SL, Rainey-Smith SR, Peiffer JJ, Xiong C, Fagan AM et al (2017) Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer's disease. Alzheimers Dement 13(11):1197-1206. https://doi:10.1016/j.jalz.2017.03.008
López-Ortiz S, Lista S, Valenzuela PL, Pinto-Fraga J, Carmona R, Caraci F, Caruso G, Toschi N et al (2023) Effects of physical activity and exercise interventions on Alzheimer's disease: an umbrella review of existing meta-analyses. J Neurol 270(2):711-725. https://doi:10.1007/s00415-022-11454-8
McAllister BB, Dyck RH (2017) Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci Biobehav Rev 80:329-350. https://doi:10.1016/j.neubiorev.2017.06.006.
Whitfield DR, Vallortigara J, Alghamdi A, Howlett D, Hortobágyi T, Johnson M, Attems J, Newhouse S et al (2014) Assessment of ZnT3 and PSD95 protein levels in lewy body dementias and Alzheimer's disease: association with cognitive impairment. Neurobiol Aging 35(12):2836-2844. https://doi:10.1016/j.neurobiolaging.2014.06.015
Adlard PA, Parncutt J, Lal V, James S, Hare D, Doble P, Finkelstein DI,Bush AI (2015) Metal chaperones prevent zinc-mediated cognitive decline. Neurobiol Dis81:196-202. https://doi:10.1016/j.nbd.2014.12.012
Baltaci SB, Unal O, Gulbahce-Mutlu E, Gumus H, Pehlivanoglu S, Yardimci A, Mogulkoc R, Baltaci AK(2022) The Role of zinc status on spatial memory, hippocampal synaptic plasticity, and insulin signaling in icv-STZ-induced sporadic Alzheimer's-like disease in rats. Biol Trace Elem Res 200(9):4068-4078. https://doi:10.1007/s12011-021-02999-2.
Whitfield DR, Francis PT, Ballard C, Williams G (2018) Associations between ZnT3, tau pathology, agitation, and delusions in dementia. Int J Geriatr Psychiatry 33(8):1146-1152. https://doi:10.1002/gps.4908
Hancock SM, Portbury SD, Gunn AP, Roberts BR, Bush AI, Adlard PA (2020) Zinc transporter-3 knockout mice demonstrate age-dependent alterations in the metalloproteome. Int J Mol Sci 21(3):839. https://doi:10.3390/ijms21030839
Martel G, Hevi C, Friebely O, Baybutt T, Shumyatsky GP (2010) Zinc transporter 3 is involved in learned fear and extinction, but not in innate fear. LearnMem 17:582–590. https://doi:10.1101/lm.1962010
Sindreu C, Palmiter RD, Storm DR (2011) Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc Natl Acad Sci USA 108:3366–3370. https://doi:10.1073/pnas.1019166108
Suh SW, Won SJ, Hamby AM, Yoo BH, Fan Y, Sheline CT, Tamano H, Takeda A, Liu J (2009) Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J Cereb Blood Flow Metab 29:1579–1588. https://doi:10.1038/jcbfm.2009.80
Nakashima AS, Butt RH, Dyck RH (2011) Alterations in protein and gene expression within the barrel cortices of ZnT3 knock out mice: experience-independent and-dependent changes. Neurochem Int 59:860–870. https://doi:10.1016/j.neuint.2011.08.007
Nozaki C, Vergnano AM, Filliol D, Ouagazzal AM, Le Goff A, Carvalho S, Reiss D, Gaveriaux-Ruff C et al (2011) Zinc alleviates pain through highaffinity binding to the NMDA receptor NR2A subunit. Nat Neurosci 14:1017–1022. https://doi:10.1038/nn.2844
Havel PJ, Uriu-Hare JY, Liu T, Stanhope KL, Stern JS, Keen CL, Ahrén B (1998) Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol 274:1482–1491. https://doi:10.1152/ajpregu.1998.274.5.R1482
Oscai LB, Molé PA (1975) Laboratory techniques involving small animals in exercise physiology research. Exerc Sport Sci Rev 3:351-370.
Jiménez-Maldonado A, Virgen-Ortiz A, Melnikov V, Rodríguez-Hernández A, Gamboa-Domínguez A, Montero S, Muñiz-Murguía J, Lemus M et al (2017) Effect of moderate and high intensity chronic exercise on the pancreatic islet morphometry in healthy rats: BDNF receptor participation. Islets 9(1):1-10. https://doi:10.1080/19382014.2016.1260796
Uchiyama M, Miharama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86 (1):271-278. https://doi:10.1016/0003-2697(78)90342-1
Kim JW, Chae J, Nam SM, Kim YN, Yoo DY, Choi JH, Jung HY, Song W et al (2015) Treadmill exercise prevents diabetes-induced increases in lipid peroxidation and decreases in Cu,Zn-superoxide dismutase levels in the hippocampus of Zucker diabetic fatty rats. J Vet Sci 16(1):11-16. https://doi:10.4142/jvs.2015.16.1.11
Sadeghi A, Hami J, Razavi S, Esfandiary E, Hejazi Z (2016) The effect of Diabetes Mellitus on apoptosis in hippocampus: cellular and molecular aspects. Int J Prev Med 7:57. https://doi:10.4103/2008-7802.178531
Chatterjee S, Peters SA, Woodward M, Mejia Arango S, Batty GD, Beckett N, Beiser A, Borenstein AR et al (2016)Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysisof 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 39(2):300-307. https://doi:10.2337/dc15-1588
Xu W, Liu J, Ma D, Yuan G, Lu Y, Yang Y (2017) Capsaicin reduces Alzheimer-associated tau changes in the hippocampus of type 2 diabetes rats. PLoS One 12(2):e0172477. https://doi:10.1371/journal.pone.0172477
Rebai R, Jasmin L, Boudah A (2017) The antidepressant effect of melatonin and fluoxetine in diabetic rats is associated with a reduction of the oxidative stress in the prefrontal and hippocampal cortices. Brain Res Bull 134:142-150. https://doi:10.1016/j.brainresbull.2017.07.013
Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PI Jr (2016) Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 473(24):4527-4550. https://doi:10.1042/BCJ20160503C
Ishida T, Takechi S (2016) Nrf2-ARE-dependent alterations in zinc transporter mRNA expression in HepG2 cells. PLoS One 11(11):e0166100. https://doi:
Smidt K, Rungby J (2012) ZnT3: a zinc transporter active in several organs. Biometals 25(1):1-8. https://doi:10.1007/s10534-011-9490-x
Olesen RH, Hyde TM, Kleinman JE, Smidt K, Rungby J, Larsen A (2016) Obesity and age-related alterations in the gene expression of zinc-transporter proteins in the human brain.Transl Psychiatry 6(6):e838. https://doi:10.1038/tp.2016.83
Kandimalla R, Thirumala V, Reddy PH (2017) Is Alzheimer's disease a Type 3 Diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis 863(5):1078-1089. https://doi:10.1016/j.bbadis.2016.08.018
Xing ZK, Du LS, Fang X, Liang H, Zhang SN, Shi L, Kuang CX, Han TX, Yang Q (2023) The relationship among amyloid-β deposition, sphingomyelin level, and the expression and function of P-glycoprotein in Alzheimer's disease pathological process. Neural Regen Res 18(6):1300-1307. https://doi:10.4103/1673-5374.358607
Lippi SLP, Smith ML, Flinn JM (2018)A Novel hAPP/htau mouse model of Alzheimer's disease: Inclusion of APP with tau exacerbates behavioral deficits and zinc administration heightens tangle pathology.Front Aging Neurosci 10:382. https://doi:10.3389/fnagi.2018.00382
Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, Hershfinkel M (2011) The neurophysiology and pathology of brain zinc. J Neurosci 31:16076–16085. https://doi:10.1523/JNEUROSCI.3454-11.2011
Sensi SL, Paoletti P, Bush AI, Sekler I (2009) Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci 10(11):780-791. https://doi:10.1038/nrn2734
Deshpande A, Kawai H, Metherate R, Glabe CG, Busciglio J (2009) A role for synaptic zinc in activity-dependent Abeta oligomer formation and accumulation at excitatory synapses. J Neurosci29(13):4004-4015. https://doi:10.1523/JNEUROSCI.5980-08.2009
Downloads
Published
How to Cite
License
Copyright (c) 2023 European Journal of Therapeutics

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The content of this journal is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Funding data
-
Selçuk Üniversitesi
Grant numbers 18202032

















